Abstract
INTRODUCTION
The continuous refinement of transplant techniques has lead to a reduction of transplant-related toxicity resulting on an increasing number of allogeneic hematopoietic cell transplantation (alloHCT) performed in older patients. Since 2014, post-transplant cyclophosphamide (PTCy) with tacrolimus (PTCy-TK), alone, has been progressively implemented at our Institution as GvHD prophylaxis for related, matched and mismatched unrelated donor transplantation (MRD, MUD, MMUD). The experience has proved that the use of this prophylaxis induces effective GvHD prevention without increased relapsed rates (Pedraza et al, 2021).
Secondary to the encouraging results obtained from the use of PTCy-TK at our Institution, and considering that older patients with hematological disorders are a group of patients with higher risk to develop transplant-related toxicity, this study compares, as far as we know, the results provided by the use of PTCy-TK with conventional GVHD prophylaxis in a consecutive cohort of patients older than 50 years.
METHODS
Between January 2014 and June 2020, 147 adults with hematological malignancies and > 50 years underwent alloHCT either from MRD or UD at our Institution. Seventy-two (48.9%) patients received PTCy 50 mg/kg/day IV on day +3 and +4, followed by TK, initiated at a dose of 0.03/kg/24h IV on day +5 and titrated to achieve a therapeutic level of 5-15mg/mL. Other GvHD prophylaxes combined calcineurine inhibitors combined with methotrexate, mycophenolate mofetil, or sirolimus, and anti-thymocyte globulin was added especially when MMUD were selected. Data were collected retrospectively and updated in June 2021. Overall survival (OS) and GvHD-Free/Relapsed free survival (GRFS) were considered the main outcome variables, and the cumulative incidence of GvHD was calculated accounting relapse and dead as competing events. In order to analyze the independent impact of PTCy-TK prophylaxis on OS and GRFS, a multivariate Cox regression analysis was performed including GvHD prophylaxis, Disease Risk Index, and transplant year (dichotomized with a cut-off in 2017, given the marked increase of PTCy-TK after this year) as explanatory variables together with other variables with prognostic value in the univariate analysis.
RESULTS
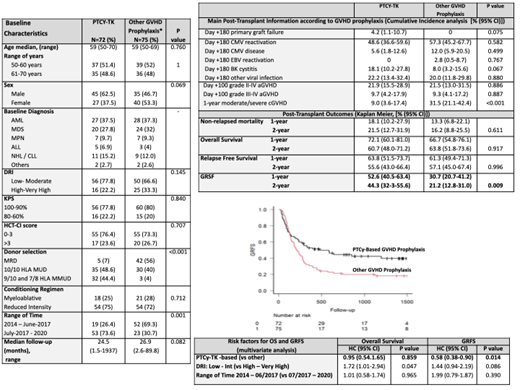
Baseline characteristics of patients classified according to the GvHD prophylaxis are reported in Figure 1. The two cohorts of patients according to GvHD prophylaxis are well balanced. Peripheral blood was the predominant stem cell source in the vast majority (97%). Of note, 53 out of 72 patients receiving PTCy-TK were transplanted between July 2017 and December 2020. And UD was used in more than 90% of PTCy-TK alloHCT, compared to 44% of alloHCT with other prophylaxes.
The median of days for neutrophil (20 vs 16, p<0.01) and platelet (19 vs 11, p<0.01) engraftment were higher for patients receiving PTCy-TK, while the differences between the incidences of viral reactivations and infections were not statistically significant between the two groups. The cumulative incidence of grade II-IV aGvHD (day +100: 21.9% vs 21.5%, p=0.88) and grade III-IV aGvHD (day +100: 9.2% vs 9.3%, p=0.88) were comparable between both cohorts, but the use of PTCY-TK resulted on a significant reduction on the incidence of moderate/severe cGvHD (1-y: 9% vs 31.5%, p<0.01) (Figure 1).
OS (1-y: 72.1% vs 66.7%, HR 0.98; p=0.91), NRM (1-y: 18.1% vs 13.3%, HR 1.20; p=0.63), and relapse rates (1-y: 18.1% vs 22.9%, HR 0.86, P=0.65) were similar in both groups (PTCy-TK and other GvHD prophylaxis, respectively). However, PTCy-TK significantly resulted into an improved GRFS (1-y: 52.6% vs 30.7%, HR 1.68, p=0.01). A multivariate analysis confirmed the independent favorable impact of PTCy-TK prophylaxis on GRFS (HR 0.58, p=0.01), but not on OS (Figure 1).
CONCLUSIONS
PTCy-TK, alone, is an effective GVHD prophylaxis for alloHCT when related and UD are selected. The use of this innovative combination provides superior GRFS than the use of conventional GvHD prophylaxis in older adults undergoing alloHCT, with comparable transplant-related mortality and relapse rates. GRFS is a composite endpoint considered a surrogate outcome of health-related quality of life, and the improvement of this parameter is remarkable in PTCy-TK alloHCT, especially for older patients.
Lozano: Grifols: Honoraria; Terumo BCT: Honoraria, Research Funding; Macopharma: Research Funding. Rosinol: Janssen, Celgene, Amgen and Takeda: Honoraria. Esteve: Novartis: Consultancy, Research Funding; Abbvie: Consultancy; Astellas: Consultancy; Jazz: Consultancy; Novartis: Research Funding; Pfizer: Consultancy; Bristol Myers Squibb/Celgene: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal